An Introduction to the Building Blocks of HVAC Absorption Chiller Operation
Many users of recreational vehicles (RVs) are familiar with refrigerators that can operate on either electricity or propane. Having utility diversity like this enables RV users to regulate potentially limited electricity and propane supplies, to meet utility demands while traveling, and make the best use of utility availability while docked. In a similar manner, many large commercial buildings are cooled with low-temperature water produced by water chillers that operate on electricity, natural gas, steam, or hot water. Like RV users, utility diversity enables facility owners to select fuels based on availability, economy, efficiency, the environment, and to address emergency conditions. Utility diversity also extends into the industrial sector in petrochemical processing, oil refining, and power generation facilities, including renewables, such as wind, hydroelectric, and solar technologies.
Utilizing the Absorption Process
So how do we cool products in RV refrigerators, environmental conditions in commercial buildings, and industrial processes with propane, natural gas, steam, hot water, and other fuels that contain heat energy? The short answer to this question is utilization of the absorption process, which was developed in the mid-1800s by a French engineer, Ferdinand Carre.
What is the Absorption Process?
Often referred to as absorbers, RV refrigerators, HVAC absorption chillers, acid gas absorbers, and membrane gas absorbers are among hundreds of absorption process applications utilized in the consumer, residential, commercial, and industrial sectors. To understand this technology, first, consider your everyday home air conditioner. Your home air conditioner operates on the vapor compression refrigeration cycle. In this cycle, electricity is utilized to power a compressor that compresses and circulates refrigerant through four basic processes: compression, condensing, expansion, and finally evaporation as shown below.
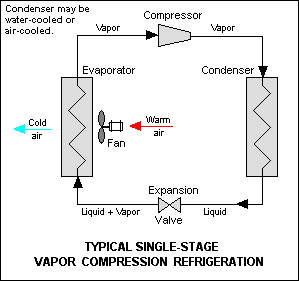
Source: Wikipedia
What happens to heat energy?
Without getting into the nitty-gritty details of each process, there is one important scientific principle that one must first understand before putting it all together. Transcending physics, chemistry, and thermodynamics, the principle is that whenever matter changes its phase, heat energy is liberated. This process takes place at a constant temperature. This means that when we convert a solid to a liquid, or a liquid to a gas, heat is absorbed by the matter undergoing the phase change. Conversely, when we convert a gas to a liquid, or liquid to a solid, heat is released by the matter undergoing the phase change. This is because, during phase changes, molecular bonds are either broken or established, depending on the direction of the phase change. Either way, it takes a lot of energy to do this!
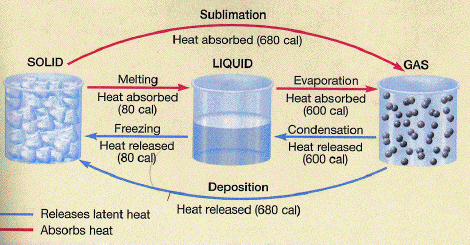
Source: John C. Butler University of Houston
How does an air conditioner work?
In the inside unit of your everyday home air conditioner, a blower circulates room air through a filter and across an evaporator coil. The evaporator coil cools the room air by indirectly exposing the room air to refrigerant in a partial gaseous but mostly liquid form. Heated by the room air, the liquid refrigerant evaporates and extracts (absorbs) heat from the room air, making the room air colder. Now in a gaseous state, the refrigerant proceeds to the outdoor unit where it’s compressed and makes its way to the condensing coil as a hot gas. Similar to the evaporator coil, the condenser coil heats the outside air by indirectly exposing the outside air to the gaseous refrigerant. Cooled by the outside air, the gaseous refrigerant condenses to a liquid and releases heat to the air, making the outside air hotter. In short, your air conditioner removes heat from your home and rejects the heat to the atmosphere by changing the phases of refrigerant from a liquid to a gas and back to a liquid again in a continuous cycle.
Commercial Building HVAC Applications
In the application of HVAC water chillers for commercial buildings, absorbers operate like vapor compression cycles and incorporate compression, condensing, expansion, and evaporation processes. However, absorbers utilize multiple components to achieve the compression process, which considerably adds to the task of explaining a myriad of refrigerant streams, simultaneous and complex process steps, and the incorporation of a carrier fluid that works in tandem with the refrigerant.
Source: Trane Technologies
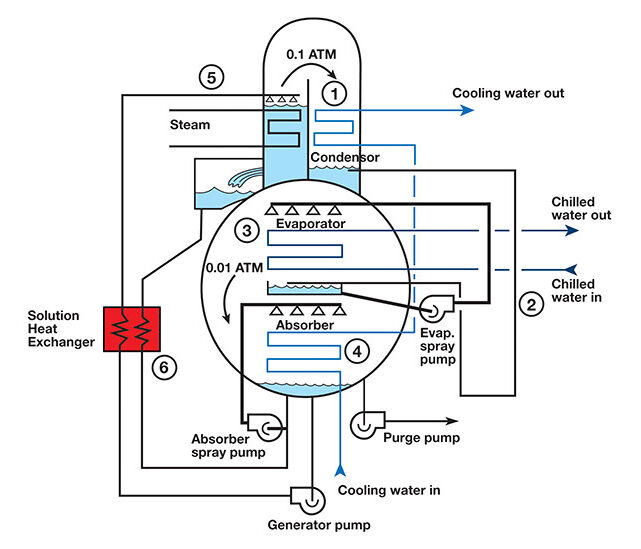
Source: Engineered Systems Magazine
How Pressure Affects Fluids
Again, without getting into the details of this sophistication, there is another important scientific principle that one must know to forge a fundamental understanding of absorber technologies. This principle is that boiling and condensing temperatures of fluids vary as a function of the pressures exerted on them. Most of us are aware that at sea level, the boiling temperature of water is 212°F. However, at the surface of the Dead Sea, the lowest elevation on earth, water boils at about 215°F. If you were to climb to the top of Mount Everest, you would find that water boils at about 158°F.
So, what does elevation have to do with boiling point? Consider the earth’s atmosphere as analogous to our oceans. As you descend into the ocean, water pressure increases, due to the weight of the water column above you. Our atmosphere is similar in that as we ascend, air (atmospheric) pressure decreases, as the weight of the air column above us decreases. This decrease upsets the pressure balance between the atmosphere and the internal pressure of the water, resulting in more rapid boiling. So, water will boil more readily and at a lower temperature at the top of Mount Everest due to reduced atmospheric pressure. In our daily life activities, we may not think of this every day, but we are like fish swimming in the ocean. However, our ocean is made up of air, which is invisible and is much less dense than water. We are reminded of this fact when driving our cars with the windows down and when it is windy.
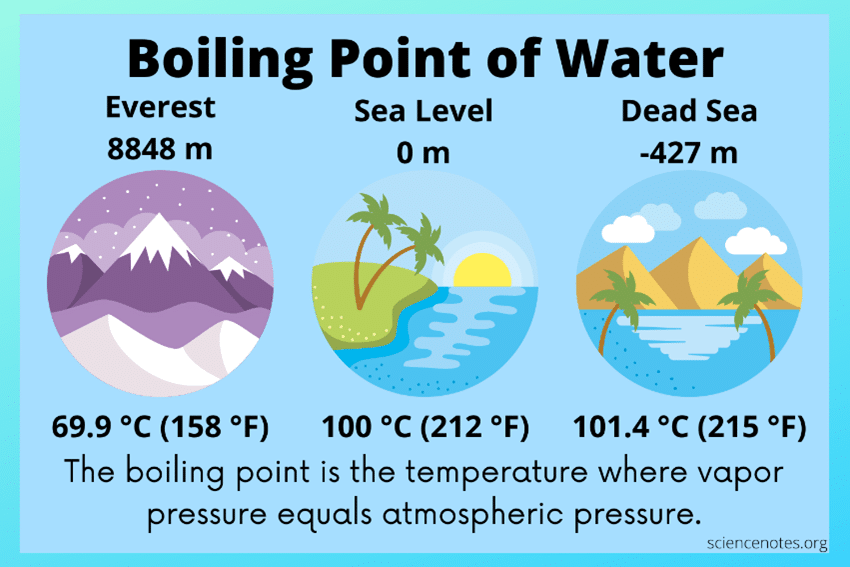
Source: Science Notes and Projects
Fluids and internal components of absorbers operate under vacuum conditions. As such, the fluids and components are exposed to pressures far lower than atmospheric pressure. Under such conditions, evaporation and other processes operate at significantly lower temperatures. This is where conditions are far more counterintuitive than what we are accustomed to in everyday life. Typically, water evaporates (boils) at 40°F in an absorption water chiller under vacuum conditions. Heat, in the form of natural gas combustion, steam, or hot water, is utilized to separate fluids, such as water (refrigerant) and lithium bromide (carrier chemical) through evaporation under vacuum conditions. In this sense, an absorber utilizes heat energy to separate fluids, change phases, and initiate the refrigeration process, which involves many other downstream process steps. Some electricity is utilized to provide liquid circulation, instrumentation, and controls. However, most of the input energy to absorbers comes from heat sources, such as natural gas, steam, or hot water. There are few moving parts in these amazing machines.
The Takeaway
So, our understanding of how we cool with heat begins with comprehension of two fundamental scientific principles – 1) during matter phase changes, heat is liberated at a constant temperature, and 2) boiling points of liquids vary as a function of external pressure conditions.
To learn more about VERTEX’s Forensic Engineering services or to speak with an Engineering Expert, call 888.298.5162 or submit an inquiry.
Author: John Imperatore



